With increasing environmental awareness, the packaging industry is seeing a growing demand for sustainable, environmentally friendly, and efficient packaging materials. Polyvinyl alcohol (PVA), as a highly water-soluble polymer, has gradually become an indispensable material in the packaging industry due to its excellent film-forming properties, adhesive strength, and good environmental characteristics.
1.Basic Characteristics and Advantages of Polyvinyl Alcohol (PVA)
Polyvinyl alcohol (PVA) is a water-soluble polymer obtained through polymerization, possessing extremely high film-forming properties, excellent adhesive performance, and strong heat resistance. In the packaging industry, PVA is mainly used to enhance the strength of packaging materials, improve protective performance, and enhance the sustainability of packaging products. Compared with other traditional plastic materials, PVA's environmental friendliness is particularly outstanding because it is biodegradable in the natural environment and does not cause long-term environmental pollution.
♣ In addition, PVA also has the following important advantages:
- High water solubility: PVA dissolves quickly in water and has excellent water solubility, giving it a natural advantage in the preparation of water-based coatings and films.
- Good film-forming properties: PVA can form a uniform and smooth film during the coating process, thereby improving the overall quality of the packaging material.
- Water and oil resistance: PVA has good water and oil resistance, effectively protecting the contents of the packaging from external environmental influences.
2. Applications of PVA in the Packaging Industry
♠ Oil- and Water-Resistant Packaging Materials
In food and industrial packaging, water and oil resistance are crucial. PVA materials have excellent water resistance, especially high-hydrolysis PVA (such as Elvanol PVOH 80-18), which can effectively isolate external moisture, thus keeping the packaging contents dry and safe. In addition, some PVA materials also exhibit good oil resistance, preventing oil penetration, making them particularly suitable for packaging oily foods.
Application example: For example, using a PVA coating in food packaging can ensure that the packaging surface is not affected by oil penetration, ensuring the quality and hygiene standards of the product. Furthermore, when used in paper-based packaging materials, PVA coatings can significantly improve the water resistance of the packaging and extend the shelf life of the products.
♠ Enhancing the Strength of Packaging Materials
In the packaging industry, the strength and durability of materials are important indicators of packaging quality. Polyvinyl alcohol (PVA), with its excellent bonding and film-forming properties, can effectively enhance the structural strength of packaging materials. Whether in paper, cardboard, or film materials, PVA can strengthen the material's tensile and tear resistance, thereby improving the overall load-bearing capacity and damage resistance of the packaging.
Application Example: Medium-viscosity PVA materials such as Elvanol 85-82 and ELVANOL 71-30 Polyvinyl Alcohol are commonly used as coatings for paper and cardboard, significantly improving the tensile and tear strength of the paper. This is particularly important for express packaging, protection during transportation, and the packaging of certain high-end products.
♠ Environmental Protection and Sustainability
With increasingly stringent environmental regulations, the packaging industry has a growing demand for biodegradable materials. PVA, as a biodegradable polymer, can degrade rapidly in the natural environment, avoiding the environmental pollution problems caused by traditional plastics. Using PVA as a packaging material not only ensures the efficiency of packaging functions but also effectively reduces environmental pollution, making it a green material that meets the requirements of sustainable development.
Application Example: For example, in the food packaging field, using PVA as a packaging coating material not only ensures the waterproof and oil-proof properties of the packaging but also reduces the negative impact on the environment. Especially in areas with high environmental requirements, the application prospects of PVA materials as biodegradable packaging are very broad.
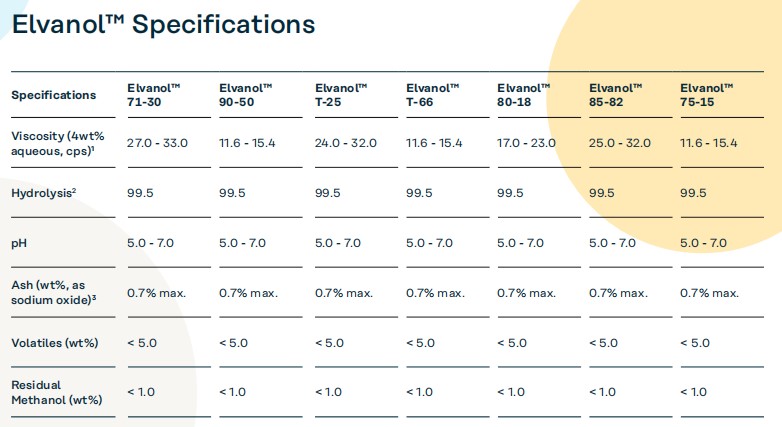
3. Specific Applications of Different PVA Grades in Packaging
Elvanol 90-50
As a high-hydrolysis, low-viscosity polyvinyl alcohol, Elvanol™ 90-50 performs excellently in packaging applications requiring lower viscosity and higher film strength. It is particularly suitable for packaging materials requiring lower viscosity and higher coating efficiency, such as primers for ceiling tiles.
Elvanol 71-30
This medium-viscosity polyvinyl alcohol is widely used in paper coating and paper packaging. Its high film-forming properties and oil and water resistance make it an ideal choice for many packaging applications. Especially in the production of paper packaging and adhesives, it can significantly improve the stability and protective capabilities of packaging materials.
Elvanol T-25 and Elvanol T-66
These two PVA grades are widely used in the textile industry, but they are also suitable for certain special packaging applications, especially in high-humidity environments, maintaining high weaving efficiency and requiring low additive levels, reducing common shedding and maintenance needs during the weaving process.
Polyvinyl alcohol (PVA), with its excellent film-forming properties, superior bonding strength, and water and oil resistance, has become an indispensable material in the packaging industry. With increasing environmental requirements, PVA, with its biodegradability and environmentally friendly characteristics, has also become a highly favored green packaging material on the market. In the future, with continuous innovation in PVA technology and the expansion of its applications, it will play an even greater role in the packaging industry, making a positive contribution to improving packaging quality and reducing environmental burden.
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com